

In a process called cryoprotection, simple sugar molecules are produced in response to desiccation or freezing by a number of species of plants and animals specifically adapted to survive extreme cold or drought [ 1 ]. The mechanism of this protective activity has been much debated, with many theories invoking a direct or indirect interaction with elements of cells such as proteins and membrane bilayers. However, the fact that a number of these sugars, such as the disaccharides sucrose and trehalose, have unusually high glass transition temperatures suggests that dynamical properties may also play a contributing role in cryoprotection. For this reason, information about the dynamics of sugars could be of considerable interest for understanding cryoprotection.
The technique of quasielastic neutron scattering ( Q E N S ) can probe motions such as molecular diffusion and rotation on the nanosecond to picosecond timescales, depending on the spectrometer. The wave vector and energy dependences of the Q E N S signal provide information about the geometry of the motions as well as rates of diffusion. Glucose is an appropriate subject for these studies since it is the canonical sugar prototype as well as a basic monomeric component of sucrose and trehalose. The present work [ 2, 3 ] focuses on the effects of the solute and solvent molecules through the choice of different energy windows and resolutions using the NCNR backscattering ( HFBS ) and disk-chopper spectrometer ( DCS ) instruments.
Selective H:D isotope substitution is a valuable tool for sorting out the dynamics of a complex organic system with Q E N S. Since the total bound-atom scattering cross section for H ( 81.7 b ) is much larger than that of D ( 7.6 b ) and most other atoms, the measured intensity is dominated by the scattering from the H atoms. Moreover the scattering from H is 98 % incoherent so that the complicating effects of interference in the scattering between different atoms can generally be neglected.
Our analysis for both the glucose and water molecule dynamics follows a model [ 4 ] that is based on the assumption that the vibrational, translational and rotational degrees of freedom are dynamically independent. In practice, this assumption results in a scattering function that is the sum of two Lorentzians, one representing translational and the other rotational motion. The latter gives rise to a peak too broad to be accurately fit in the energy windows measured in this experiment. In the incoherent approximation the rapid jump diffusion model for the translational Lorentzian has a full width at half maximum ( FWHM ) given by: 2
t = 2
DQ² / ( 1 + D
0Q² ) , where D is the diffusion constant and
0 is the period between jumps. The two Lorentzians were convolved with an instrumental resolution function to fit the measured spectra.
Since the dynamics of water molecules have been found to be generally much faster than those of sugar molecules, the motion of the two can be separated effectively through the judicious choice of energy window and energy resolution of the instrument. To explore the dynamics of the glucose molecules, the sugar, in which the exchangeable hydrogen atoms had been pre-deuterated, was dissolved in D2O and studied on HFBS with an energy window of ±36 μe V. Even though the coherent scattering cross section of water is comparable to the incoherent cross section of glucose, the faster dynamics of the water results in a broad contribution to the observed signal that does not contribute to the translational motion lineshape.
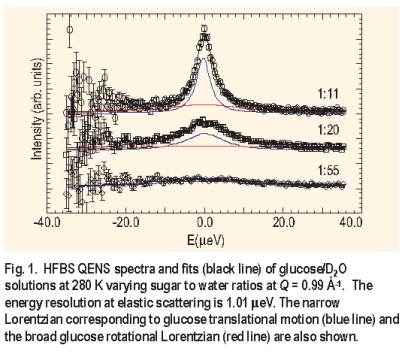
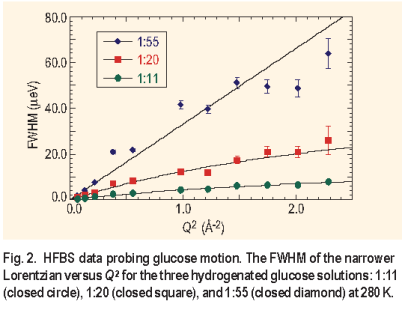
Three aqueous glucose solutions were studied at 280 K with glucose to water ratios of 1:11, 1:20, and 1:55. Two Lorentzians were used to fit the spectra ( Figure 1 ), although the rotation component was generally too broad to be accurately fit in the given energy window. The effect of concentration on the glucose translational motion is evident. The narrow Lorentzian reduces in width with increasing glucose concentration as the diffusive motion slows. The FWHM for the two highest concentrations was found to follow the random jump diffusion model ( Figure 2 ) while the lowest concentration manifested the characteristics of continuous diffusion:
r =
DQ². As the concentration of the glucose increases, the diffusion rates decrease by almost a factor of 6 from 25.1 ± 0.2 x 10-7 cm 2/s for the 1:55 ratio solution to 4.5 ± 0.1 x10-7 cm 2/s for the 1:11 solution.
The larger energy window and energy resolution (
= 110 μe V ) available on DCS permitted the examination of the water dynamics in aqueous glucose solution to the exclusion of the glucose dynamics. The slower dynamics of the glucose as observed on HFBS results in the signal primarily contributing to an elastic peak on DCS. To further minimize the incoherent contribution from the glucose, the unexchangeable hydrogen atoms on the molecule were replaced by deuterium atoms. Again three different aqueous glucose solutions were studied with the same ratios as on HFBS. In addition to the fully deuterated glucose, glucose deuterated only at the C6 carbon atom ( d 6 6 g l u) and deuterated glucose with a methoxy group at the C2 carbon atom ( m g l u ) were studied at the various concentrations. The full width at half maximum of the narrow Lorentzian from the fit, corresponding to the translational motion of the water molecules, had a Q2 dependence typical of the random jump diffusion model ( Figure 3 ).
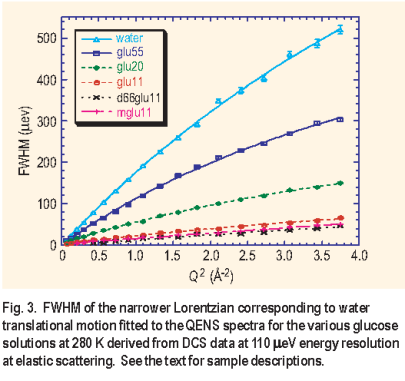
The diffusion rate dropped by a factor of 7 from 1.08 ± 0.02 x 10 -5 cm 2/s for the 1:55 solution to 0.16 ± 0.01 x 10 -5 cm 2/s for the 1:11 solution. A significant difference in diffusion constant was not observed for solutions that had glucose with deuterium substitutions, further justifying the static approximation for glucose motion on DCS.
Both the glucose and water have similar degrees of change in the translational diffusion constants, reflecting the effect of hydrogen bonding on dynamics. Interaction with glucose thus disrupts the organization of the water molecules, as the retarding effect is observed for glucose to water ratio as low as 1:55. These results hint at the role that sugar may play in cryopreservation: in dry environments the binding of waters by sugar may prevent local dessication, while in cold environments the sugar disruption of the water network may prevent local destruction by favoring glass rather than ice formation.
[1] F. Franks Biophysics and Biochemistry at Low Temperatures; Cambridge University Press: Cambridge (1985).
[2] L. J. Smith, J. W. Brady, Z. Chowdhuri, D. L. Price and M.-L. Saboungi, submitted to J. Chem. Phys.
[3] C. Talon, L. J. Smith, J. W. Brady, J. R. D. Copley, D. L. Price, M.-L. Saboungi, submitted to J. Phys. Chem.
[4] J. Teixiera, M.-C. Bellisant-Funel, S.-H. Chen, and A. J. Dianoux, Phys. Rev. A. 31, 1913 (1985).
L. J. Smith1, 2, C. Talon3, J. W. Brady4, Z. Chowdhuri5, 6, J. R. D. Copley5, D. L. Price7, M.-L. Saboungi3
1 Argonne National Laboratory, Argonne, IL 60439
2 Clark University, Worcester, MA 01610
3 Centre de Recherche sur la Matiere Divisee, 5071 Orleans Cedex 2, France
4 Cornell University, Ithaca, NY 14853
5 NIST Center for Neutron Research
National Institute of Standards and Technology
Gaithersburg, MD 20899-8562
6 University of Maryland, College Park, MD 20742
7 Centre de Recherche sur les Materiaux a Haute Temperature 45071 Orleans Ce
Back to FY2003 HTML main page
Go to previous article
Go to next article
To view all symbols correctly, please download Internet Explorer 6 or Netscape 7.1