Biomimetic membranes have been developed as models of living cell membranes, and this has applications in the quest for biocompatibility of inorganic materials in biologically active mediums, such as coatings for artificial organs. A membrane consists of a lipid bilayer ( two lipid layers ) where hydrophobic carbon chains form the inside of the membrane and their polar head groups the interface with the aqueous surrounding medium. A supported membrane-mimic consists of a lipidlike bilayer, typically attached to a single-crystal substrate, with access to water only at the top surface [ 1, 2 ]. Here we use neutron reflectometry to study a system in which water has access to both sides of a membrane-mimic attached to such a substrate, thus making the system a closer mimic to a real cell membrane.
The system devised by Liu et al. [ 3 ] consists of a water-swellable polyelectrolyte that electrostatically binds to the substrate and acts as a “cushion” for the membrane, not unlike the cytoskeletal support found in actual mammalian cell membranes. The lower half of the membranemimic is a terpolymer that attaches to the polyelectrolyte. A phospholipid layer forms on top of the terpolymer and the bilayer is finally chemically crosslinked for added stability. The system is shown schematically in Figure 1.
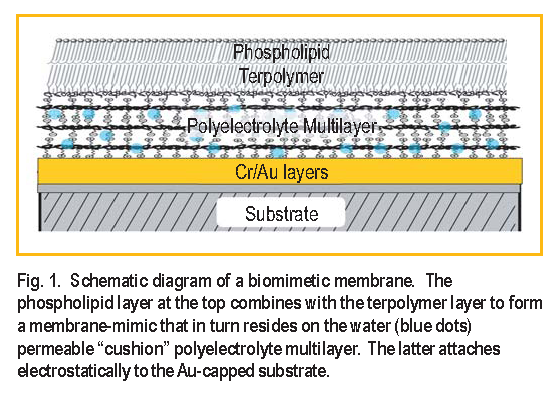
Neutron reflectivity measurements were performed at the NG-1 vertical stage reflectometer to obtain the compositional profile at every step of the assembling process of the membrane-mimic which consisted of three stages: a ) polyelectrolyte multilayer ( PE ), b ) polyelectrolyte multilayer plus terpolymer ( PE+T E R ), and c ) polyelectrolyte multilayer plus terpolymer plus phospholipid layer ( PE+T E R+PC ) [ 4 ]. The spatial resolution attained was approximately 10, about half the thickness of a membrane bilayer, making it possible to distinguish the two layers of a membrane but not the structure of a single layer
A unique compositional profile of the biomimetic film with no a priori knowledge of the sample’s composition is obtained by measuring the reflectivity of equivalent samples made onto two substrates [ 5 ]. The substrates used were single crystal silicon ( Si ) and sapphire ( Al2O3 ) coated with chromium ( Cr ) and then a gold ( Au ) layer to allow the polyelectrolytes to bind to a similar surface on both wafers.
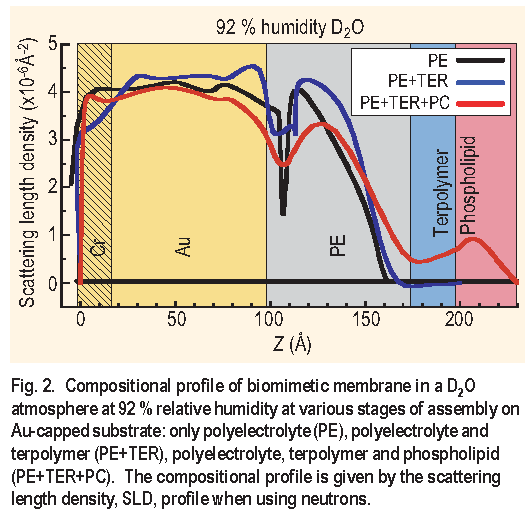
Figure 2 shows the compositional profiles for the PE,
PE+T E R and PE+T E R+PC assemblies in a D2O atmosphere
at 92 % relative humidity. The figure shows that
the hydration of the PE layer is almost unaffected by the
addition of the terpolymer and the phospholipid layer. Also,
upon the addition of the phospholipid layer to the PE+T E R
assembly, the composite PE+T E R+PC assembly shows an
increase in thickness of approximately 30  consistent
with the formation of a single phospholipid layer at the
surface. It is also clear that the addition of a phospholipid
layer onto the terpolymer layer rearranges this region
significantly, since the terpolymer layer only becomes
apparent after the phospholipid layer is added. It is possible
to verify with an independent technique ( contact angle )
that the terpolymer was in fact deposited because it forms
a hydrophobic outer layer. The outer surface becomes
hydrophilic once the phospholipid layer is deposited onto
the terpolymer layer.
consistent
with the formation of a single phospholipid layer at the
surface. It is also clear that the addition of a phospholipid
layer onto the terpolymer layer rearranges this region
significantly, since the terpolymer layer only becomes
apparent after the phospholipid layer is added. It is possible
to verify with an independent technique ( contact angle )
that the terpolymer was in fact deposited because it forms
a hydrophobic outer layer. The outer surface becomes
hydrophilic once the phospholipid layer is deposited onto
the terpolymer layer.
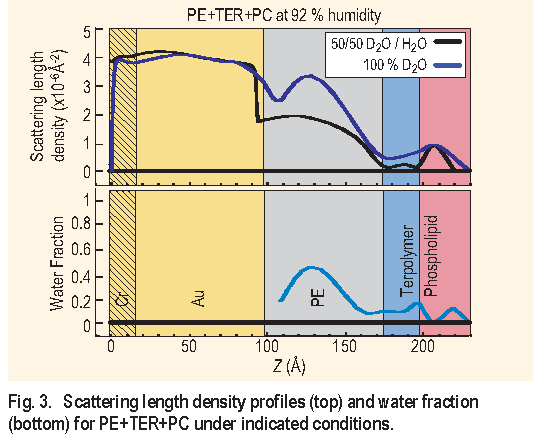
Figure 3 ( top ) shows the profile for the PE+T E R+PC
assembly under 92 % relative humidity in 100 % D2O and
in 50/50 D2O/H2O. The overall thickness change due to the
intake of water, in going from dry ( not shown ) to 92 %
relative humidity, was found to be 20 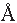 . Figure 3 ( bottom )
shows the water fraction in the assembly under 92 %
relative humidity. This is obtained by assuming that the
distribution of each component in the layers is unaffected
by having either D2O or 50/50 D2O/H2O. From the figure it
can be seen that the polyelectrolyte multilayer has a 40 %
water uptake. This is a significant amount of water, which
suggests that the polyelectrolyte multilayer can work as a
“cushion” for membrane-mimetic systems. The terpolymer
and the phospholipid layers contain an average of 10 %
water, which is also significant, suggesting that these
layers are not tightly packed.
. Figure 3 ( bottom )
shows the water fraction in the assembly under 92 %
relative humidity. This is obtained by assuming that the
distribution of each component in the layers is unaffected
by having either D2O or 50/50 D2O/H2O. From the figure it
can be seen that the polyelectrolyte multilayer has a 40 %
water uptake. This is a significant amount of water, which
suggests that the polyelectrolyte multilayer can work as a
“cushion” for membrane-mimetic systems. The terpolymer
and the phospholipid layers contain an average of 10 %
water, which is also significant, suggesting that these
layers are not tightly packed.
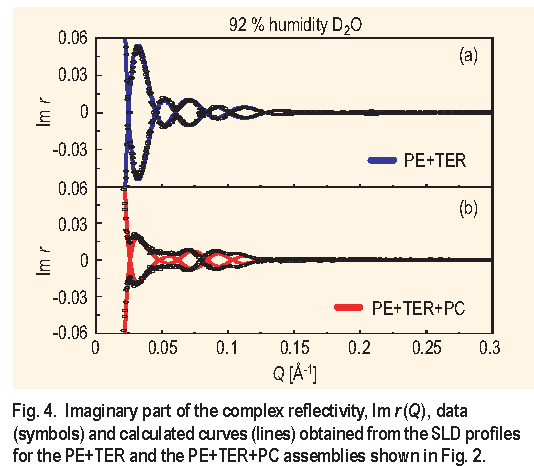
The method of making equivalent samples on two substrates to obtain a unique compositional profile has a built-in congruency test, particularly useful in checking the reproducibility of the samples as well as the quality of the films. The test is to compare the calculated imaginary part of the complex reflectivity from the obtained profile with the corresponding data, as is shown in Figure 4 for the PE+T E R and PE+T E R+PC assemblies. From Figure 4 it is concluded that the PE+T E R samples are homogenous and essentially identical while for the PE+T E R+PC assembly, the absence of true zeros, as indicated by the calculated curve, is suggestive of a small degree of sample inhomogeneity.
The system from Liu et al. has many characteristics desirable in a biomimetic membrane. It is a single membrane- mimic attached to a significantly hydrated soft “cushion” support that allows some membrane proteins to function. Thrombomodulin, a membrane protein relevant to blood-clotting, is being studied in this membrane-mimic environment to further develop biocompatible coatings for artificial organs [ 6 ].
References:
[1] E. Sackmann, Science 271, 43 (1996).
[2] A. L. Plant, Langmuir 15, 5128 (1999).
[3] H. Liu, K. M. Faucher, X. L. Sun, J. Feng, T. L. Johnson, J. M. Orban, R. P. Apkarian, R. A. Dluhy, E. L. Chaikof, Langmuir 18, 1332 (2002).
[4] U. A. Perez-Salas, K. M. Faucher, C. F. Majkrzak, N. F. Berk, S. Krueger, E. L. Chaikof, Langmuir 19, 7688 (2003).
[5] C. F. Majkrzak, N. F. Berk, U. A. Perez-Salas Langmuir 19, 1506 (2003).
[6] J. Feng, P. Y. Tseng, K. M. Faucher, J. M. Orban, X. L. Sun, E. L. Chaikof, Langmuir 18, 9907 (2002).
U. A. Perez-Salas
NIST Center for Neutron Research
National Institute of Standards and Technology, Gaithersburg, MD 20899-8562
K. M. Faucher
Emory University School of Medicine, Atlanta, GA 30322
C. F. Majkrzak, N. F. Berk, S. Krueger
NIST Center for Neutron Research
National Institute of Standards and Technology
Gaithersburg, MD 20899-8562
E. L. Chaikof
Emory University School of Medicine
Atlanta, GA 30322