The presence of structural disorder can have a dramatic effect on macroscopic materials properties. At the atomic scale, structural disorder is typically described in terms of distributions of geometrical quantities such as bond lengths and bond angles that are determined from diffraction measurements. Alternatively, disorder can be quantified by determining the distribution of potential energies experienced by the atomic scale constituents of the system. The distribution is sharp for a highly ordered (crystalline) system, while it is broad for a disordered system. Rotational tunneling spectroscopy is a useful tool for characterizing the local molecular environment due to its exquisite sensitivity to the local potential (Refer to reference 1). This approach has been used to determine the distribution of rotational potentials felt by methyl iodide molecules adsorbed in a series of mesoporous silica glasses. The degree of confinement-induced disorder in the methyl iodide can then be directly related to the pore sizes in the glass.
The rotational potential felt by the methyl (C H sub 3) groups in methyl iodide (C H, sub 3, I,) arises solely from interactions with the local environment including the neighboring molecules. The energies of the transitions between rotational states can be found by solving the Schrödinger equation for the model potential by V sub 3 times, open parenthesis, one minus cosine of three theta, close parenthesis, over 2, in the angular coordinate about the carbon iodine axis, theta. In the limit of an infinitely high barrier, V sub 3, the motion of the C H sub 3 group is that of a torsional oscillator. However, if the barrier is not too large, the wave functions will have significant overlap and quantum mechanical tunneling between the potential minima can occur. This results in a splitting of the rotational ground state. The energy of this splitting, which can be directly measured using cold neutron spectroscopy, has a nearly exponential dependence upon the barrier height. This strong dependence on V sub 3 is the reason why tunneling spectroscopy is so sensitive to the local molecular environment and, in particular, the quenched disorder of adsorbed molecular solids.
The porous hosts used in this investigation have a range of pore size distributions with differing nominal pore diameters and widths. Nominal pore diameters, d average, determined via nitrogen adsorption/desorption isotherms range from 2.5 nanometers to 14.4 nanometers. In all cases but one (xerogel with d average = 10.0 nanometers) the porous glasses are disks. The xerogel is a fine powder with a nominal particle size of 11 micro meters. Liquid methyl iodide (C H, sub 3, I,) was condensed into the porous hosts until 95 % of the open pore volume was filled and then cooled to 5 K.. Spectra were collected using the N I S T High-Flux Backscattering Spectrometer with an energy resolution of 0.79 micro electron volts.
The neutron scattering spectra display broad asymmetric lines indicative of a distribution of potential barrier heights. These distributions can be extracted from the neutron scattering data using a simple model in which a sum of Gaussian distributions of potential barriers (which yield an asymmetric tunneling line-shape) is used to fit the spectra (Refer to reference 2). A sum of two potential distributions is found to be sufficient to describe the data. One of these distributions is quite broad, indicating substantial disorder of the methyl iodide solid, while the other is much narrower suggesting a relatively high degree of order.
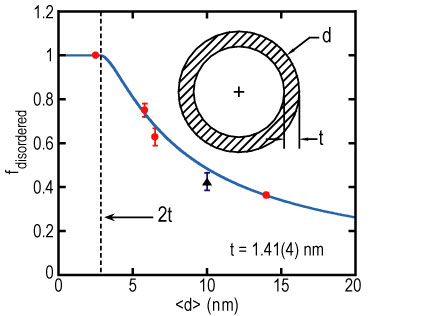
| Figure 1. Fraction of the total integrated intensity that appears in the disordered component of the tunneling spectrum as a function of the mean pore diameter. The solid line is a fit to the model discussed in the text. The dashed line is drawn at twice the disordered layer thickness. Inset shows the geometrical picture used in the model. |
The relative weights of the two components show a pronounced dependence on d average. Figure 1 shows that the fraction of the total integrated intensity that appears in the highly disordered component of the tunneling spectrum decreases monotonically as d average increases. This behavior can be described with a simple model in which the relatively well-ordered component of the scattering is attributed to C H, sub 3, I, molecules near the center of the pore while the highly disordered component is associated with a ring of thickness t of disordered molecules near the pore walls. Treating the pores as circular cylinders, the fraction of disordered molecules is just the ratio of the ring to circular areas: f sub disordered = 4 times t times, open parenthesis, d average minus t, close parenthesis, over d average squared.
The solid line in Figure 1 compares this model to the data for t = 1.41 open parenthesis, 4, close parenthesis nanometers, which corresponds to about three molecular layers. It is clear that this model provides an excellent fit to the data.
While the relative weights of the two components depend on the pore size, the mean values, V sub 3 average, and widths, sigma sub V 3, of the two distributions of barrier heights are found to be roughly the same for each of the hosts. This indicates that the structure of the confined methyl iodide both near the surface and in the center of the pores does not depend strongly on either d average or the width of the pore size distribution.
C H, sub 3, I, molecules possess a relatively large electric dipole moment. This results in the molecules being anti-aligned in the bulk crystal structure (Refer to reference 3). Silica surfaces often have hydroxyl (O H) groups which would be expected to interact rather strongly with the dipole moment of the molecules and lead to a substantial surface-molecular interaction. The surface-molecule and molecule-molecule interactions can be of similar strength leading to structural frustration in the confined solid. Thus, one would expect that the disordered fraction and even the structure of the two components to be strongly influenced by changing the relative strengths of these interactions. In order to test this hypothesis, the surface of one set of porous disks (d average = 5.8 nanometers) was chemically treated such that hydrophobic O C H sub 3 groups replaced the O H groups. This change significantly reduces the surface-guest interaction, and should therefore lead to a reduction of the disorder in the confined solid. The measured tunneling spectra for methyl iodide adsorbed in both the treated and untreated hosts (d average = 5.8 nanometers) are shown in Figure 2. As seen by the prominence of the narrower peaks, the more ordered component of the tunneling spectrum increases substantially for the methyl iodide confined in the glass with the hydrophobic surface as compared to the host with the hydrophilic O H groups. In fact, the disordered fraction decreases by about 25 %, which indicates that the thickness of the highly disordered layer is roughly two-thirds that of the untreated glass. Moreover the width of the tunneling line in the highly disordered component has also decreased substantially indicating that the structure of the methyl iodide near the hydrophobic surface is more bulk-like than it is near the hydrophilic surface.
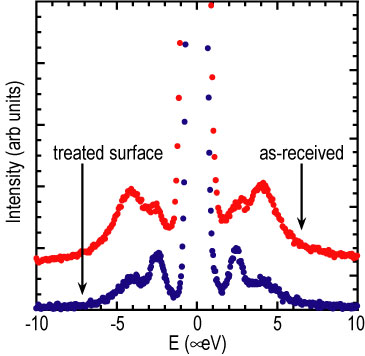
| Figure 2. Tunneling spectrum of confined methyl iodide, in 5.8 nanometer pores with and without surface treatment to make the surface hydrophobic. The hydrophobic surface results in a diminished disordered component. |
In summary, the sensitivity of tunneling spectroscopy to small changes in the rotational potential has allowed us to probe the importance of surface-guest interactions in determining the disorder imposed by the confinement of methyl iodide within mesoporous glasses. It has been shown that the adsorbed species in the hydrophilic pores form a highly disordered layer with a thickness of approximately three molecular layers and that a more ordered region exists in the pore center. Interestingly, the molecular structures of these two regions do not depend strongly on either the average pore size or on details of the pore size distribution. When the surface chemistry is altered so as to reduce the importance of surface-guest interactions, both the thickness and molecular scale disorder of the region near the wall are greatly reduced.
References
[1] W. Press, in Single-Particle Rotations in Molecular Crystals, (Springer-Verlag, Berlin, 1981).
[2] R. M. Dimeo and D. A. Neumann, Phys. Rev. B 63, 014301 (2001). R. M. Dimeo and D. A. Neumann, App. Phys. A 75 (in press). R. M. Dimeo, D. A. Neumann, Y. Glanville, and D. B. Minor, Phys. Rev. B 66, 104201 (2002).
[3] M. Prager, J. Stanislawski, and W. Hausler, J. Chem. Phys. 86, 2563 (1987).
Authors
R. M. Dimeo and D. A. Neumann
NIST Center for Neutron Research
National Institute of Standards and Technology
Gaithersburg, MD 20899-8562
Y. Glanville
The Pennsylvania State University
University Park, PA 16802-6300
D. B. Minor
Ceramics Division
National Institute of Standards and Technology
Gaithersburg, MD 20899-8520
Back to FY2002 HTML main page
Go to next article
To view all symbols correctly, please download Internet Explorer 6 or Netscape 7.1